About Amoxicillin
Each year an estimated 1.36 million children die due to pneumonia alone. Despite the existence of simple, inexpensive treatment such as amoxicillin, only 30% of children with pneumonia receive an antibiotic.
Commodity Introduction
An estimated 1.36 million children die each year due to pneumonia alone. Pneumonia is an infection that causes the lungs to fill with pus and fluid, which makes breathing difficult and limits oxygen absorption. Pneumonia pathogens can be transmitted through the air, blood or during delivery in the birth canal.[1] Risk factors that make children more susceptible to pneumonia include: inadequate nutrition and a lack of zinc, vaccine-preventable disease (e.g. measles, pertussis), HIV and tuberculosis infection, diarrhea, low birth weight, non-exclusive breastfeeding in first six months, indoor air pollution, lack of sanitation and crowded living conditions.[2] Guidelines from WHO for pediatric medicines recommend amoxicillin as the gold standard treatment for non‐severe pneumonia in children under five.
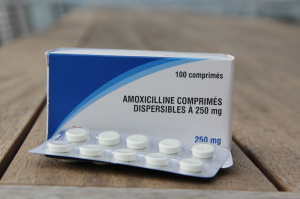
Photo courtesy of UNICEF
Commodity Context
Non-severe pneumonia among children under five can be treated with simple antibiotics. Antibiotics, such as amoxicillin, can prevent the majority of pneumonia deaths and cost only about $US 0.21-0.42 per treatment course. Despite the existence of this simple, inexpensive treatment, many children in need are often left behind: only 27% of children with pneumonia receive an antibiotic.[1] In many cases, children receive inappropriate, and ineffective, medicines because health workers are not familiar with or disagree with standard treatment protocols and do not comply with national guidelines to correctly treat childhood pneumonia. Program managers report that often health providers are unaware that the pediatric formulation of the antibiotic recommended for pneumonia treatment is available at an affordable price.[3]
Products Available
The WHO recommends dispersible antibiotics as the most convenient formulation for children. Amoxicillin is prepared in 250mg scored, dispersible tablet (DT) in a blister pack of 10 DTs. The market for DT is more developed in Asia, where many brands include Amoxi DT in 125 mg and 250 mg. Still, the majority of products in the market are suspension and capsules. Cost of DT is much lower than that of powder for oral suspension. The average cost per treatment course is approximately USD $0.23-0.44 for children 2-11 months and US$0.46 to 0.63 for children 12-59 months.
Product Efficacy
WHO has gathered evidence on amoxicillin and is updating official treatment guidelines to include amoxicillin DT as first line treatment for children under age five. A randomized control trial comparing three day vs. five day treatment with amoxicillin for non-severe pneumonia in young children concluded that treatment with oral amoxicillin for three days was as effective as for five days in children with non-severe pneumonia.[4] An evidence summary from Ghana Essential Medicines Committee also confirmed dispersible amoxicillin offers significant benefits over suspensions in terms of cost, product stability and ease of transport.[5]
Common Barriers
At the individual level, knowledge gaps are common among health care providers regarding the causes and severity of pneumonia, and effective treatments.
Although women may be socially identified as care providers, in some contexts their ability to treat children is dependent on their husband’s approval to seek care or spend money on treatment. The lack of male involvement in childcare and lack of community health education about pneumonia are therefore barriers to care seeking.
Amoxicillin and antibiotic distribution is usually limited to a certain trained cadre of health providers, limiting access and distribution/administration possibilities at the community level. There are also product packaging challenges given the difference in administration for children younger than 1 years old vs. those aged 1-5 year olds.
For more information on amoxicillin, please visit the UN Every Woman Every Child initiative webpage.
References
- UNICEF; WHO. (2006). Pneumonia: The forgotten killer of children.
- WHO. (2013). The Global Action Plan for Pneumonia and Diarrhea.
- PSI. (2013). The DELTA Companion: Marketing Planning Made Easy.
- ISCAP Study Group. (2004). Three day versus five day treatment with amoxicillin for non-severe pneumonia in young children: a multicentre randomised controlled trial. BMJ: British Medical Journal, 328(7443), 791.
- Daniel Kwame Afriyie; Cynthia Amaning Danquah; Kwame Ohene Buabeng. (2011). Evidence Summary: Dispersible amoxicillin tablets for children.


 This website is made possible by the support of the American People through the
This website is made possible by the support of the American People through the